To store hypochlorous acid effectively and maximize its shelf life its critical to keep it away from any direct or indirect light sources, particularly UV rays and to store it in an ideal temperature range of 4 to 25°C to ensure that it remains effective for use in sanitization and disinfection applications.
As individuals who work with hypochlorous acid day in and day out we’re we understand how crucial proper storage is to maintain the potency and efficacy of HOCl.
Through our guidance and storage tips, you’ll be guaranteed to extend the shelf life of HOCl, ensuring every drop is as potent as it can be.
Key Takeaways
- Store hypochlorous acid (HOCl) in a cool, dark place (4–25°C) to maintain its potency.
- UV light and direct sunlight degrade HOCl, reducing its effectiveness.
- Use opaque, coated glass containers to prevent light exposure and contamination.
- Improper storage can cause HOCl to lose concentration (ppm) and efficacy.
- Do not mix HOCl with other substances, as it may destabilize the solution.
- Signs of HOCl degradation include loss of its faint pool-like smell and reduced disinfection power.

The Importance of Proper Hypochlorous Acid Storage
We often emphasize the extraordinary capabilities of hypochlorous acid (HOCl) as a sanitizer, cleaner, and disinfectant thanks to its numerous beneficial properties.
To maintain these properties and ensure the substance performs effectively in various applications, it’s crucial to understand both the properties that make HOCl so versatile and the challenges associated with its storage.
Understanding Hypochlorous Acid’s Unique Properties
Hypochlorous acid is a powerhouse with substantial biocidal activity, perfect for eradicating pathogens across a broad spectrum. The naturally occurring HOCl’s non-toxic nature is of utmost importance for safe use in diverse environments, from domestic kitchens to school classrooms.
Its potent disinfectant properties are owed to its ability to structurally destroy harmful bacteria and viruses, providing a holistic cleaning solution without the accompanying risks of harsh chemicals.
Read more on how hypochlorous acid is formed and the science behind it.
Common Challenges in Hypochlorous Acid Storage
Hypochlorous acid, despite its multitude of uses, is not without its storage challenges. One of the central battles we face is its instability; HOCl is particularly sensitive to UV light exposure and contamination with other substances.
These elements can precipitate a decline in its concentration (ppm), mitigation of its disinfection efficacy, and impurity of the solution.
Which simply put, means if you need your Hypochlorous acid to work as well as it can and not have it become useless within just a month or two then carefully managing how and where HOCl is stored is vital for you to understand.
At HOCL Hub we sell pure and stable Hypochlorous acid in bulk at the best wholesale rates in the UAE for industries looking to cost effective disinfection or cleaning solutions.
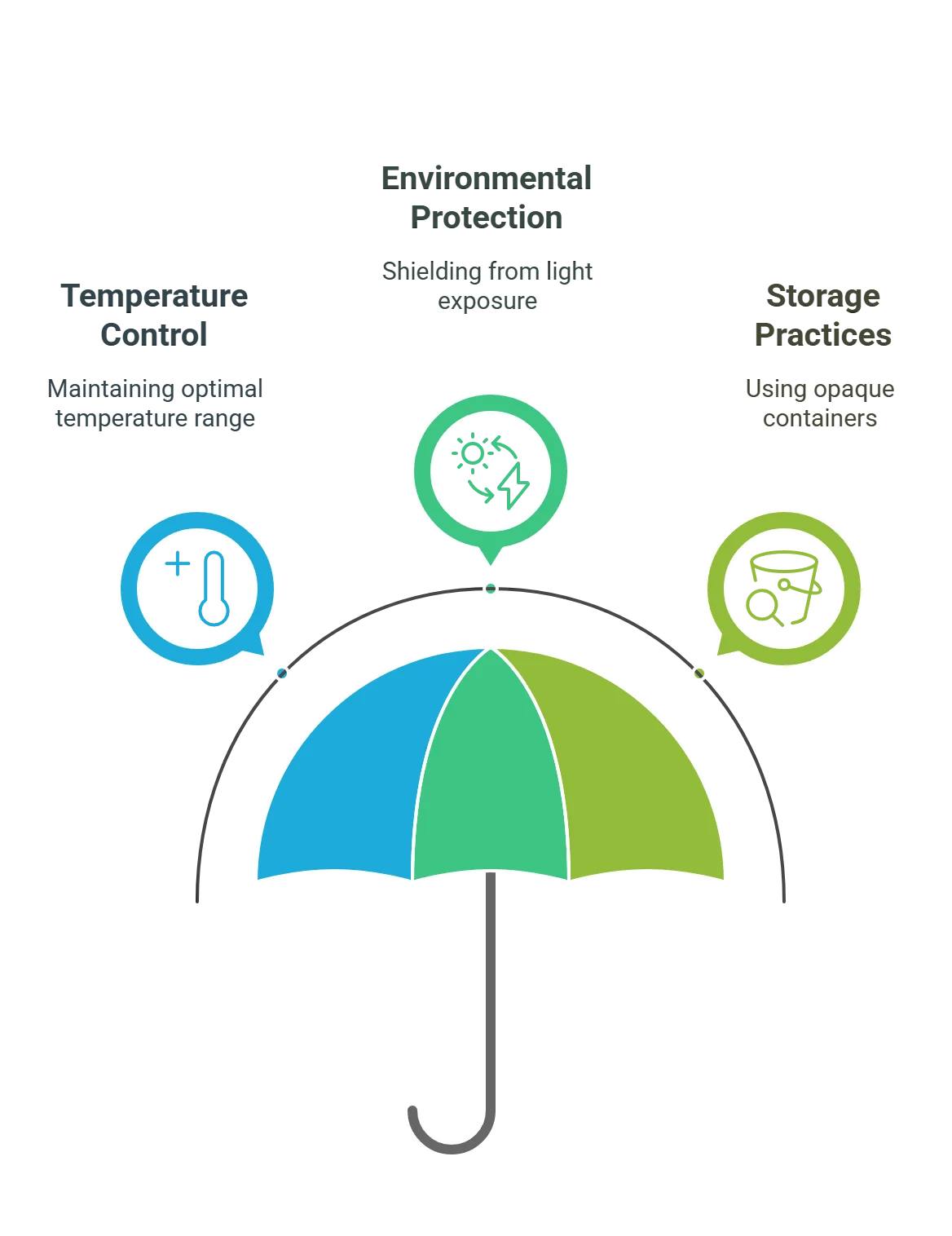
Optimal Conditions to Store Hypochlorous Acid
Understanding and implementing the optimal storage conditions for hypochlorous acid is essential for preserving its stability and efficacy. By adhering to recommendations regarding temperature and environment, you can significantly enhance the longevity of this potent sanitizing agent.
- Ideal Temperature
- For hypochlorous acid, a stable temperature is key to conservation. It’s crucial to maintain storage conditions within a temperature range of 4 to 25°C to preserve product purity and prevent efficacy loss. Brief excursions to temperatures between 25 to 40°C may be tolerated; however, such fluctuations should be minimized to ensure product stability.
- Environmental factors
- exposure to sunlight and UV rays can degrade the hypochlorous acid molecule, so it must be stored in an environment that protects it from light-induced decomposition.
Best Practices for Minimizing Light Exposure
To minimize light impact and maintain hypochlorous acid stability, certain best practices should be implemented:
- Always store HOCl in opaque containers or the original coated glass bottles provided by the brand you’ve bought your HOCl from, to shield against UV rays and sunlight.
- Avoid the storing the HClO solution into clear or transparent containers, as this can increase the risk of light-induced degradation.
- When transporting or using HOCl outside, make sure it remains in its protective packaging to prevent unnecessary light exposure.
- Keep storage areas for HOCl dark and away from windows or other sources of direct sunlight.
If you follow these guidelines, we can ensure that your hypochlorous acid will remain as effective as when you bought it ensuring maximum shelf life.
Do All Hypochlorous Acid Solutions Have the Same Shelf Life?
Not all hypochlorous acid solutions are created equal. While proper storage is crucial, the initial shelf life of HOCl can vary significantly between different manufacturers and formulations.
The key factors that influence how long your hypochlorous acid solution will remain stable and effective are as follows.
Initial Concentration (PPM)
The starting concentration of HOCl plays a vital role in its stability. Higher concentrations (500+ ppm) typically degrade faster than solutions with moderate concentrations (200-300 ppm).
This is because higher concentrations create more opportunities for decomposition reactions.
Based on the scientific data available, solutions between 200-300 ppm often provide the optimal balance between effectiveness and stability.
pH Level and Stabilization
The pH level is perhaps the most critical factor in HOCl stability. At a pH between 3.5-6.5, hypochlorous acid exists predominantly in its most effective form (HOCl).
However, as referenced in several safety data sheets, even slight pH changes can cause the solution to convert to less effective forms like hypochlorite ions (OCl-).
Modern stabilization techniques used by leading manufacturers can help maintain this crucial pH balance.
Production Method
The way HOCl is produced significantly impacts its shelf life:
Single-Cell Electrolysis: This modern production method creates more stable HOCl solutions with shelf lives up to 12 months when properly stored. The process results in fewer contaminants and better pH control.
Traditional Split-Cell: Older production methods often yield solutions with shorter shelf lives (3-6 months) due to more variability in the final product.
Water Quality
The purity of water used in production matters immensely:
- Deionized Water: Creates more stable solutions
- Tap Water: Can introduce minerals and contaminants that reduce shelf life
- Filtered Water: Falls somewhere in between, depending on filtration quality
Additional Factors Affecting Stability
Several other elements can impact HOCl shelf life:
- Packaging Material: Dark, UV-resistant containers protect against degradation better than clear ones.
- Manufacturing Environment: Clean room conditions during production reduce contamination risks.
- Quality Control: Rigorous testing and standardization processes ensure consistent product stability.
- Transportation Conditions: Exposure to temperature extremes during shipping can impact shelf life even before you receive the product.
Remember to always check the manufacturer’s expiration date and storage recommendations, as these are based on their specific formulation and stability testing.
Sultan Alam Khan leads HOCl Hub as Editor in Chief, where he oversees product reviews and guides on cleaning solutions. Specializing in hypochlorous acid applications and green cleaning technology, he helps readers choose effective, eco-friendly disinfection products.
His expertise spans sustainable sanitization methods, chemical safety, and environmental health. At HOCl Hub, Sultan ensures all content meets strict editorial standards for accuracy and scientific backing.